Introduction
The median age for DLBCL diagnosis is 66 years. Incidence increases with age and about 30% of patients diagnosed are over 75 years old. In the context of an aging population, unfit or frail individuals pose a unique challenge due to their limited tolerance for combination chemotherapy and are often excluded from clinical trials. The VHA is one of the largest integrated providers of cancer care in the United States catering to an older population, making it ideal to study real-world outcomes in this geriatric population. This study presents the final analysis of our large cohort of geriatric patients (≥ 65 years old) diagnosed with DLBCL treated within the VHA.
Methods
This study reviewed 6266 randomly selected patients with an ICD code for DLBCL within the VHA between January 1, 2011, and December 31, 2021. Patients were excluded if they had a diagnosis other than DLBCL, incomplete records, or diagnosed and/or treated outside of the VHA. Data abstractors collected baseline patient and disease characteristics and treatment responses. Fall and delirium rates,Charlson Comorbidity Index and survival rates were determined via electronic health record query. Patient population ≥ 65 years old were divided into three age groups: 65 to 74 years, 75 to 84 years, and ≥ 85 years, represented by A, B, and C, respectively. Chi-square tests were used to analyze the relationship between age and variables of interest.
Results
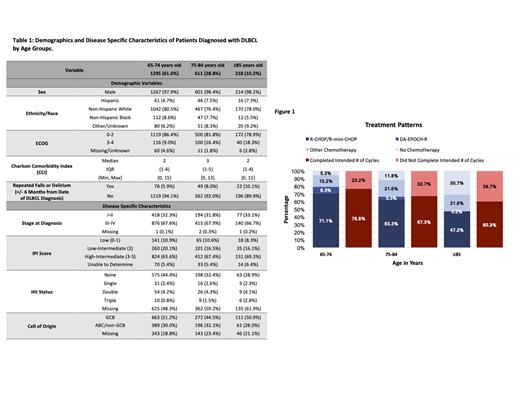
Of the total population 3178, 2124 (66.8%) qualified for data analysis due to the age cut of ≥ 65 years. The distribution of age was 61.0%, 28.8%, and 10.2% in A, B, and C, respectively. The demographics and clinical characteristics of the patients are shown in Table 1. 18.3% of patients in C had an ECOG score 3-4 compared to 16.4% in B and 9.0% in A. With increasing age, patients had an increased incidence of repeated falls and delirium within the six months prior to and following diagnosis (5.9% in A vs 8.0% in B vs 10.1% in C).
Across all age groups, R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone) remained the most common first-line regimen. The utilization of R-CHOP decreased significantly from A to C (69.7% vs 53.5% vs 27.5% in A, B, and C, respectively). In comparison to the other age groups, patients in group C were more likely to receive R-mini-CHOP (1.5% in A vs 9.8% in B vs 19.7% in C). As shown in Figure 1, the percentage of patients that did not receive any chemotherapy increased with age (5.3% vs 11.8% vs 30.7% in A, B, and C, respectively, p=<0.001). Of those who received chemotherapy, 23.2% in A, 32.7% in B, and 39.7% in C did not complete the intended number of treatment cycles. 693 (32.6%) patients did not receive any treatment or were not able to complete the intended number of cycles. Among patients who received treatment, older patients were more likely to have primary refractory disease or die before completing the first line of treatment (21.8% vs 24.4% vs 34.8% for A, B, and C, respectively, p=0.001). Additionally, geriatric patients with primary refractory disease were less likely to receive salvage therapy. Median overall survival was 71, 42, and 14 months in A, B, and C, respectively. The 12-month survival rate was 77% in A, 69% in B, and 51% in C. Only 1.3% of the total population were enrolled in a clinical trial.
Conclusion
This study is one of the largest to examine disease patterns, treatment approaches, and outcomes in a geriatric cohort diagnosed with DLBCL within the VHA. Low treatment initiation and completion rates decline significantly with age above 65 years. Poor performance status and increased incidence of delirium and falls lead to a low percentage of very elderly patients completing the intended number of chemotherapy cycles. Our study suggests that approximately one-third of patients are unable to receive first line treatment or are unable to complete first line intended treatment. The outcomes are dismal for patients with relapse or refractory disease which further contributes to very low long-term survivals. Novel therapies, such as antibody drug conjugates, bispecific T-cell engagers and chimeric antigen receptor T-cell therapy offer different toxicity profiles and possible increased tolerance. Nonchemotherapy-based novel agents in upfront approaches should include geriatric patients in clinical trials to ensure that the benefits of medical advancements extend to this population addressing the unique challenges they face.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal